The global induced pluripotent stem cells (iPSCs) market represents a transformative segment in regenerative medicine, disease modeling, and drug discovery. These cells, generated by reprogramming adult somatic cells to an embryonic-like state, are poised to redefine the landscape of personalized medicine. iPSCs eliminate ethical concerns associated with embryonic stem cells and offer immense potential in treating a wide array of chronic and genetic conditions. The continuous advancement in gene editing tools, bioengineering technologies, and tissue regeneration platforms is driving extensive research and investment in the field.
Full Details Report: https://www.databridgemarketresearch.com/reports/global-induced-pluripotent-stem-cells-market
Trends in the iPSCs market reveal a strong momentum in therapeutic applications, particularly in neurological, cardiovascular, and autoimmune diseases. The surge in funding from public and private entities is catalyzing stem cell research across various countries. Institutions such as the National Institutes of Health (NIH) and organizations in Japan, the U.K., and Germany are aggressively supporting regenerative medicine. Moreover, partnerships between academia and biopharma are becoming increasingly prominent, facilitating translational research and commercial product development.
Another significant trend is the integration of artificial intelligence (AI) and machine learning in cell screening, toxicity testing, and modeling of disease pathways using iPSCs. AI-enabled platforms are enhancing the predictive accuracy and efficiency of iPSC-based assays, accelerating preclinical drug testing. Meanwhile, CRISPR-Cas9 genome editing is being leveraged to correct genetic mutations in iPSCs, paving the way for precise, patient-specific therapies. The rise of 3D organoids and tissue-on-chip models built from iPSCs is also gaining traction, offering realistic simulations of human organ functions for research and therapeutic development.
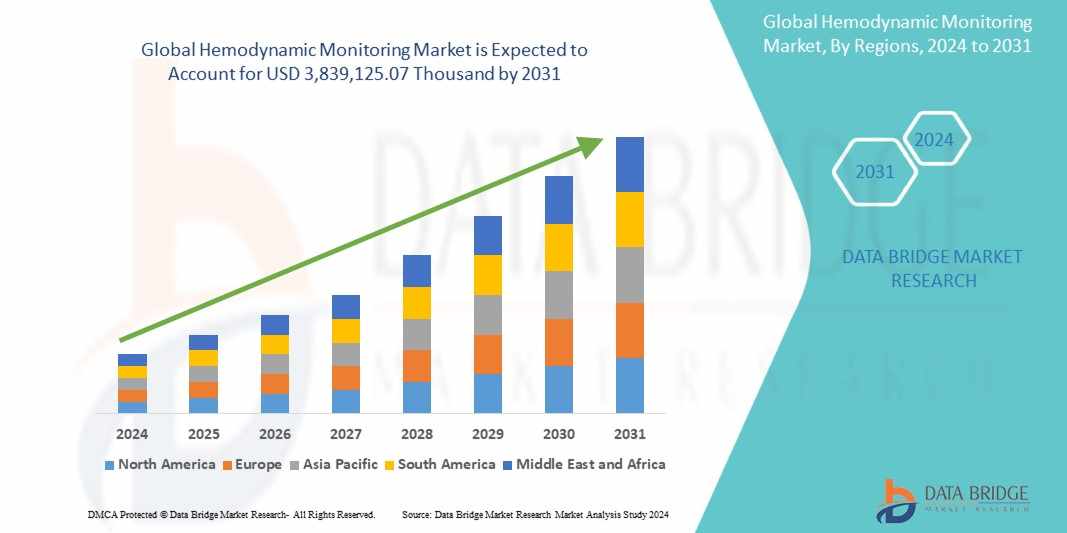
The market size for induced pluripotent stem cells is expanding rapidly. In 2024, the market was valued at approximately USD 2.3 billion and is expected to grow at a compound annual growth rate (CAGR) exceeding 8.5% during the forecast period from 2025 to 2032. This growth is underpinned by a rising global burden of chronic diseases, growing demand for cell-based therapies, and advancements in biomanufacturing processes. North America holds the largest market share, driven by robust healthcare infrastructure, advanced research facilities, and a high concentration of key market players. The U.S. alone accounts for a significant proportion of iPSC patents and clinical trials.
Latest Trending Reports:
Global Trace Minerals in Feed Market
Global Water Based Lamination Adhesive Market
Global Multivendor ATM Software Market
Global Dairy Protein Market
Global Protective Goggles for Medical Market
Global Home Medical Furniture Market
Global Paper Pulp Disposable Tableware Market
Asia-Pacific is emerging as a promising region, particularly with Japan leading in stem cell research and regulatory approval of iPSC-derived products. Japan’s regenerative medicine laws have accelerated product development and commercialization timelines. China is also witnessing growing investments in iPSC R&D, supported by government initiatives and a rising biotech ecosystem. Europe, with its strong regulatory framework and funding support, remains a vital region for iPSC research and development, especially in the U.K., Germany, and France.
Market share is dominated by biotechnology and pharmaceutical companies focusing on drug discovery and disease modeling. Companies like FUJIFILM Cellular Dynamics, REPROCELL, Ncardia, and Fate Therapeutics are playing a key role. Academic research centers and contract research organizations (CROs) are also crucial contributors, especially in iPSC line development, validation, and customization services. The demand for high-throughput screening tools and culture media tailored for iPSC growth is creating opportunities for ancillary product suppliers.
Growth in the iPSC market is fueled by a surge in collaborations and licensing agreements aimed at advancing clinical applications. Numerous iPSC-based clinical trials are underway, targeting diseases like Parkinson’s, macular degeneration, diabetes, and spinal cord injuries. The potential to differentiate iPSCs into virtually any cell type makes them ideal for autologous therapies. As the focus shifts from bench to bedside, regulatory bodies are establishing frameworks to ensure safety, efficacy, and ethical compliance in iPSC-based interventions.
Demand for iPSCs is rising sharply due to their expanding role in personalized medicine. The ability to create patient-specific cell lines for drug toxicity testing and therapeutic screening is revolutionizing pharmaceutical R&D. In oncology, iPSCs are used to generate immune cells that can be engineered to target tumors more effectively. In cardiology, iPSC-derived cardiomyocytes offer new avenues for studying congenital heart diseases and evaluating drug responses. Moreover, the cosmetics industry is beginning to explore iPSCs for non-animal testing models and skin regeneration studies.
The future of the iPSCs market lies in scalable, GMP-compliant manufacturing processes that ensure consistent quality and safety of cell lines. Automation and bioprocessing innovations are being introduced to streamline cell reprogramming, differentiation, and expansion. As clinical trials progress and therapeutic efficacy is established, commercialization of iPSC-derived products will gain momentum. Investments in cryopreservation, cell banking, and logistics are likely to increase to support large-scale production and distribution.
Another area with immense future potential is allogeneic iPSC therapies. While autologous therapies are personalized, they are also time-consuming and expensive. Allogeneic therapies, where iPSCs are derived from healthy donors and used across multiple patients, offer scalability and cost benefits. Companies are developing universal donor iPSC lines through gene editing to reduce immunogenicity, enhancing the feasibility of off-the-shelf stem cell treatments.
The iPSC market is also expected to benefit from ongoing developments in regulatory harmonization. Efforts by international agencies to standardize quality control, safety assessment, and clinical protocols will facilitate faster approvals and wider adoption. Intellectual property protection, ethical governance, and patient consent frameworks will be critical in shaping the market’s trajectory.
In conclusion, the global induced pluripotent stem cells market is positioned for robust growth, driven by technological innovations, expanding therapeutic applications, and supportive regulatory environments. iPSCs are becoming indispensable tools in modern medicine, enabling groundbreaking approaches to treat complex diseases. As research matures into real-world therapies, the iPSC market is likely to transform from an exploratory science into a cornerstone of next-generation healthcare.
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com










Write a comment ...