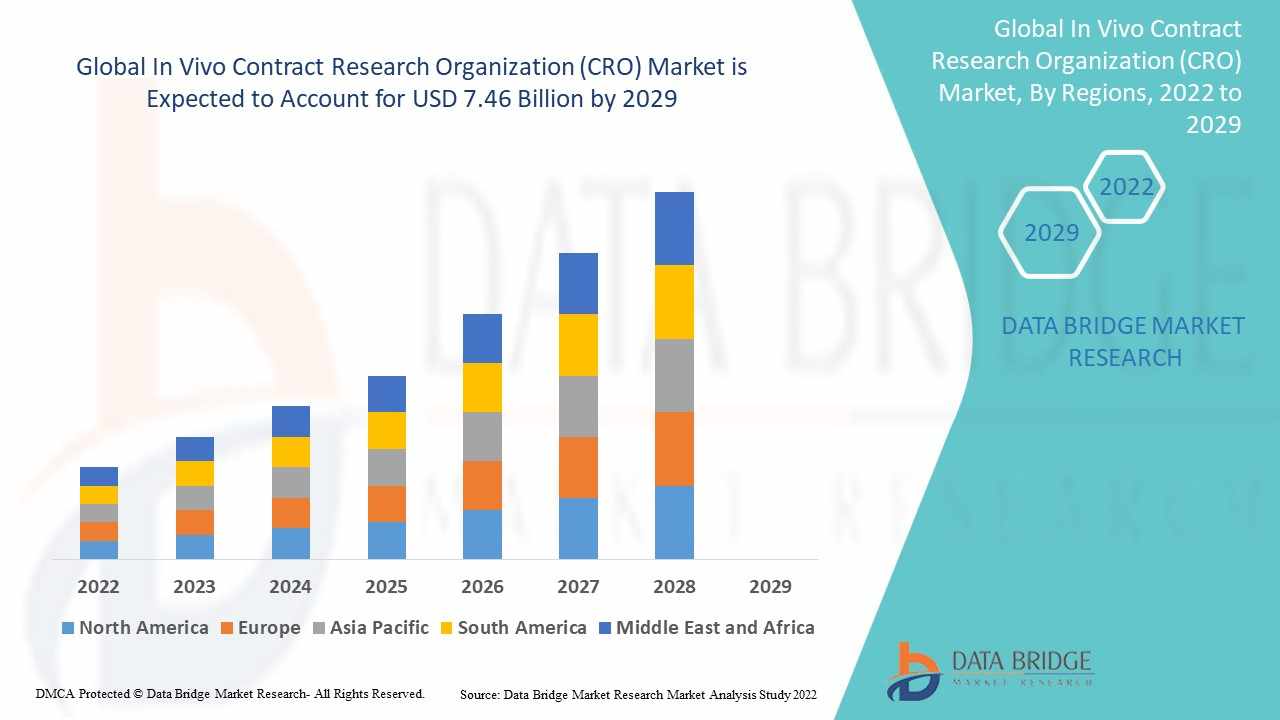
The In Vivo Contract Research Organization (CRO) market plays a pivotal role in the global pharmaceutical and biotechnology industry by offering essential services that support drug development, especially during the preclinical phase. These organizations conduct in vivo studies—research involving live animals—to determine the safety and efficacy of new therapeutic compounds before human trials begin. With growing R&D investments, an increasing demand for new drugs, and regulatory complexities, pharma and biotech firms are increasingly outsourcing to specialized CROs to optimize timelines and reduce costs.
Full Details Report: https://www.databridgemarketresearch.com/reports/global-in-vivo-contract-research-organization-cro-market
In vivo CROs offer a range of services, including pharmacology, toxicology, pharmacokinetics, and disease modeling. These services are critical in predicting human responses and identifying potential adverse effects, helping organizations make informed go/no-go decisions early in the pipeline. As a result, the in vivo CRO market has evolved into a key strategic asset in drug discovery and development.
One of the major trends shaping this market is the growing preference for outsourcing among pharmaceutical and biotech companies. Outsourcing in vivo studies to CROs offers access to experienced researchers, specialized facilities, and advanced technologies without the overhead costs associated with in-house operations. This trend is fueled by the need to shorten development timelines and maintain competitive advantages in an increasingly crowded therapeutic landscape.
Latest Trending Reports:
Global Human Milk Oligosaccharides (HMOs) in Infant Formula Market
Global Service Lifescale Management Market
Global Paper Pallets Market
Global Cross Platform Mobile Advertising Market
North America Pharmaceuticals Packaging Testing Equipment Market
Another trend gaining traction is the adoption of advanced technologies and models to improve study accuracy and efficiency. CROs are investing in high-resolution imaging systems, digital data analytics, AI-based platforms for modeling, and genetically engineered animal models. These innovations enhance data precision and enable faster decision-making, which in turn accelerates the drug development process.
The global in vivo CRO market has witnessed robust growth in recent years and is projected to continue expanding. As of 2024, the market size is estimated to be valued at USD 5.8 billion and is expected to reach approximately USD 9.6 billion by 2030, growing at a compound annual growth rate (CAGR) of 8.5%. This growth is driven by the increasing complexity of new drug molecules, regulatory pressure to validate preclinical safety, and rising demand for personalized medicine research.
North America currently holds the largest market share, attributed to a strong presence of major pharmaceutical companies, advanced research infrastructure, and a favorable regulatory environment. The U.S. remains the key driver in this region due to its robust funding for medical research and high concentration of CROs. Europe follows closely with well-established life sciences sectors in Germany, the UK, and France. Meanwhile, Asia-Pacific is emerging as a fast-growing region, driven by cost advantages, improving regulatory frameworks, and expanding pharma manufacturing capabilities, particularly in China and India.
Market share is also segmented based on service type. Toxicology studies represent the largest segment owing to the mandatory requirement of such studies by global regulatory agencies before clinical trials can begin. Pharmacokinetics and pharmacodynamics (PK/PD) studies are also experiencing high demand due to their role in understanding a drug’s absorption, distribution, metabolism, and excretion. Disease models and efficacy testing are expected to gain significant traction as more biotech firms enter the space focusing on oncology, neurology, and rare diseases.
The growth of the in vivo CRO market is underpinned by a combination of technological advancement and structural shifts in the pharmaceutical R&D ecosystem. Drug developers are under constant pressure to deliver more precise and safer medications in less time. This dynamic is pushing organizations to collaborate with CROs who bring not only operational efficiency but also domain expertise, regulatory knowledge, and scalable infrastructure.
Biotech startups and small pharmaceutical firms, which often lack in-house capabilities, are increasingly relying on in vivo CROs for end-to-end preclinical services. These partnerships help accelerate early-stage development, optimize resources, and manage financial risks associated with high R&D expenditure. The agility of CROs in adapting to project-specific requirements makes them ideal collaborators for such entities.
Demand for in vivo CRO services is also bolstered by the rising focus on oncology, autoimmune disorders, and neurodegenerative diseases. These therapeutic areas often require complex in vivo studies involving sophisticated animal models and long-term studies. As more biologics and gene therapies enter the pipeline, in vivo CROs are adapting to meet the specialized testing requirements, thus expanding their capabilities and service offerings.
The future of the in vivo CRO market looks promising with the increasing adoption of humanized animal models, integration of digital tools, and global expansion of CRO capabilities. Humanized models offer improved translational relevance, allowing researchers to better predict human outcomes and reduce failure rates in clinical trials. Digitalization of data collection and analysis helps enhance study reproducibility, regulatory compliance, and project timelines.
Investments in AI and machine learning are also reshaping how in vivo CROs operate. Predictive analytics, automated workflows, and real-time data monitoring are helping CROs deliver more precise and faster results. This tech-driven evolution will further align CRO operations with the needs of modern drug development, especially in precision medicine and immunotherapy.
However, challenges such as ethical concerns around animal testing, evolving regulatory landscapes, and the need for high capital investment continue to influence the market dynamics. Regulatory authorities are encouraging the adoption of 3Rs (Replacement, Reduction, Refinement) in animal testing, pushing CROs to adopt more humane and scientifically advanced methods. Compliance with Good Laboratory Practices (GLP) and evolving international standards adds another layer of complexity that CROs must manage effectively.
Despite these challenges, the outlook for the in vivo CRO market remains strong. Continuous innovation, global collaborations, and regulatory alignment are expected to create new opportunities. As drug development becomes more global and more specialized, the role of in vivo CROs will be further cemented as critical enablers of pharmaceutical innovation.
In summary, the In Vivo Contract Research Organization (CRO) market is at the forefront of enabling efficient and reliable preclinical drug development. Driven by outsourcing trends, technological innovation, and increasing R&D demands, the market is poised for substantial growth. As companies seek to de-risk development and expedite timelines, the value proposition of in vivo CROs will become even more essential in the evolving pharmaceutical landscape.
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com










Write a comment ...